Por Dr. Carlos Ortiz Ortiz
ABSTRACT
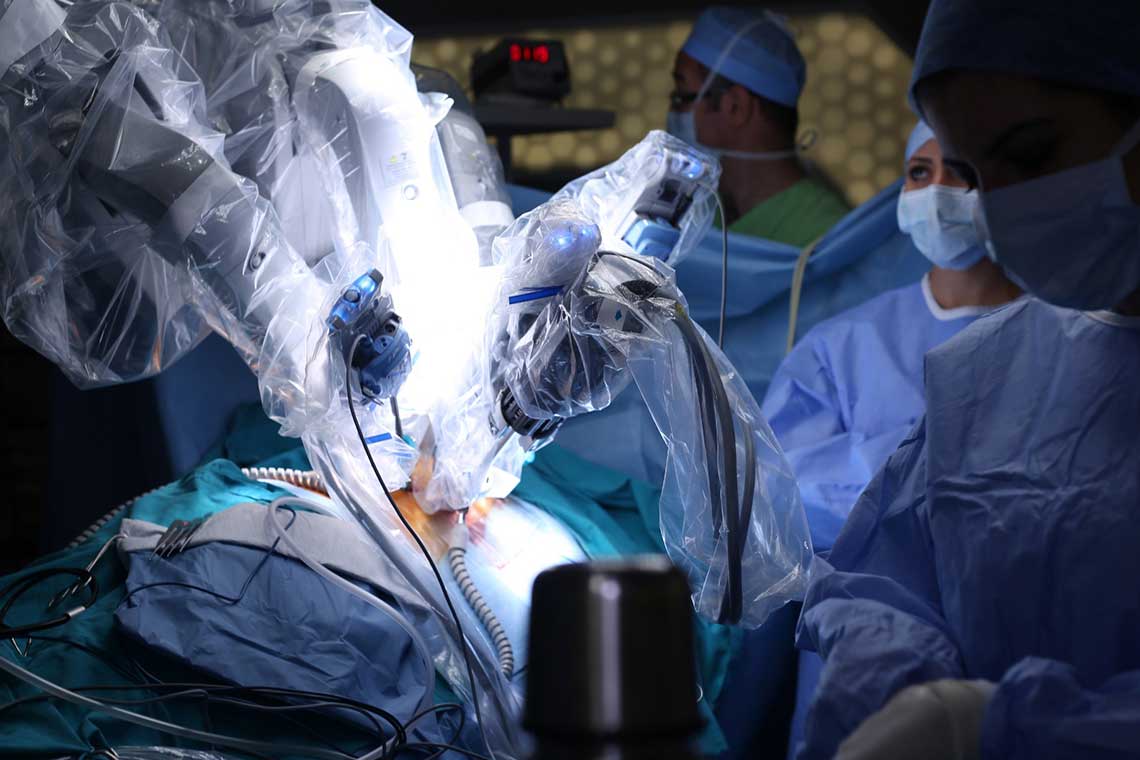
Minimally invasive surgery (MIS) has become a standard of care in the last years. It has focused on minimizing the invasiveness of surgical procedures using a small video camera and instruments through small incisions. Endoscopy technology and instrumentation have made it possible to convert many procedures in different surgical specialties.
Computer and robotics have been used for the last 25 years. Robotic surgery enhances dexterity and sensitivity combine with media images at display promises to simplify complex procedures and avoid intraoperative complications.
Surgical procedures previously performed through large and painful incisions that required long recovery times are now performed with a much shorter recovery and excellent outcomes.
The real advantage in comparison with the open approach is the low incidence of wound complications, shorter recovery time, and length of stay.
KEYWORDS
Minimally invasive surgery, Robotics, small bowel, outcomes, 3-D Imaging, NIR fluorescence imaging.
153.1 Introduction
Computer enhanced robotic systems, has multiples advantages, offering a greater range of motion stability and accuracy. It also allows for the integration of imaging, telesurgery, in addition to other multiple benefits inherent to individual surgical specialties.
Advantage of robotic versus laparoscopic in surgery is tremor free movement, less surgeon fatigue, better visualization with 3-D Imaging and ergonomics. Haptic feedback is one of the most critical disadvantages of robotic surgery, but new technology it’s been developing to overcome this hurdle.
153.1.1 Indications
Small bowel conditions such as bowel obstruction, gastrointestinal stromal tumor (GIST), deformities, bleeding ulcers caused by inflammation of the small intestine, Meckel Diverticulum, non-cancerous tumors.
Some of the more frequent indications for small bowel surgery are:
- Blockage in the intestine with adhesive Small Bowel Obstruction. Obstruction not amendable to Adhesiolysis
- Gastrointestinal stromal tumor (GIST), Noncancerous (benign) tumors and suspicious of malignance polyps.
- Malignancy: Suspected malignancies will typically require 8–10 cm margins resection.
- Non-traumatic perforation: Is an uncommon serious complication associated with high morbidity and mortality, can be originated by ulcerations of the small intestine, They have been described in conjunction with numerous diseases, including lymphoma, carcinoma, radiation enteritis, ischemia, Crohn’s disease, systemic lupus erythematosus, Henoch-Schönlein purpura, Behçet’s disease, and numerous medications.
- Necrotizing enterocolitis, Typhi ulcers, for adult patients includes ulcerative disease and malignancy, small bowel resection may be preferred over the repair to allow for tissue diagnosis by a pathologist
- Traumatic perforation: Defects can be repaired if they encompass less than 50% of the small bowel loop; otherwise, it will require resection.
- Necrosis due to malperfusion occurs secondary to thrombus, emboli, or irrigation deficit, this ischemia is frequently progressive if the causal diseases state is not corrected, often requiring multiple resections and carrying high morbidity.
- Inflammatory bowel disease (IBD): is one of the most frequent sources of small bowel sickness, resection is typically the last treatment choice, reserved for strictures not amendable to stricturoplasty or conservative management.
- Symptomatic Meckel’s diverticulum or diverticular disease
153.2 Technique
For Robotic Small Bowel Resection, atraumatic graspers are preferred to prevent serosal injury. Electrocautery or cutting devices such as the Ligasure or Harmonic Scalpel are required for mesenteric dissection. Fig. 1 and Fig. 2
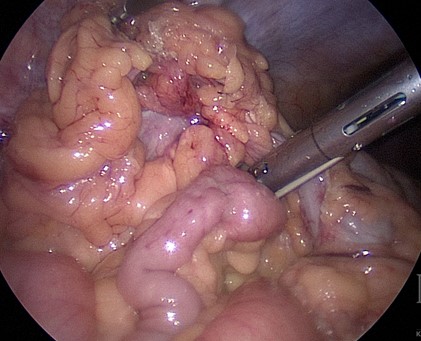
1: Power Devices
Intracorporeal prefer over extracorporeal anastomosis. Side to side anastomosis with robotic stapler gives the surgeon more angulations and laterality compared with laparoscopic stapler which is control by an assistant.
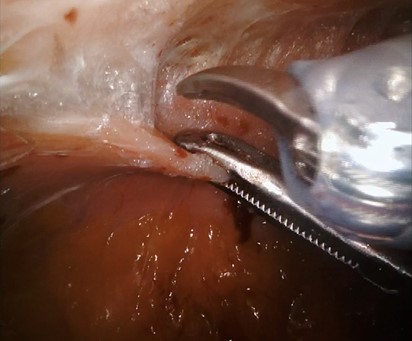
2: Atraumatic Grasper
“Blue load should be utilized to divide small intestine proximal, distal and for anastomosis. Enterotomy can be close with a stapler if there are a large lumen and surgeon not concern of possible stricture. Hand sewn intracorporeal anastomosis closure is more amenable robotic than laparoscopic for reasons above stipulated. A stitch is place proximal to anastomosis, arm 4 is use to pull stitch up aligning anastomosis. Arms 1 and 3 are for suturing.
If using 4 trocar technique Arm 1 bipolar forceps, followed by camera in arm 2. Arm 3 energy device preferable vessel sealer and arm 4, which is used for retraction and exposure, atraumatic grasper or small grasping. Instruments in arm 1 and 3 are interchangeable as needed.
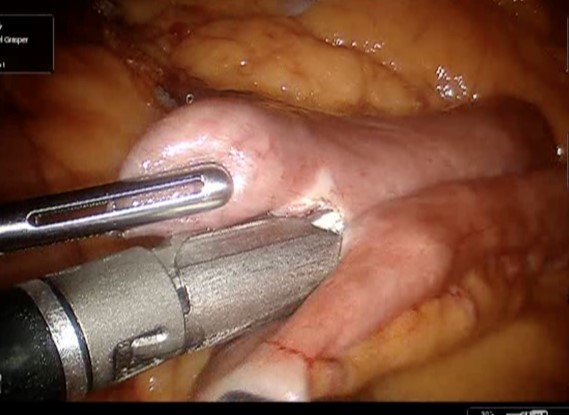
3: Robotic Stapler
Anastomotic dehiscence is the ‘Achilles heel” for postoperative surgery and a major cause of mortality. Several prospective randomized controlled studies have compared hand suturing versus mechanical stapling. Many factors contribute to wound healing and integrity of an anastomosis, such as blood supply, the tension on the anastomosis, patient comorbidities and inflammatory factors. In trauma patients level 3 evidence suggests small bowel resection follow by a sutured anastomosis will result in significantly fewer leaks and intra-abdominal abscess than to stapled technique ( Fig. 3).
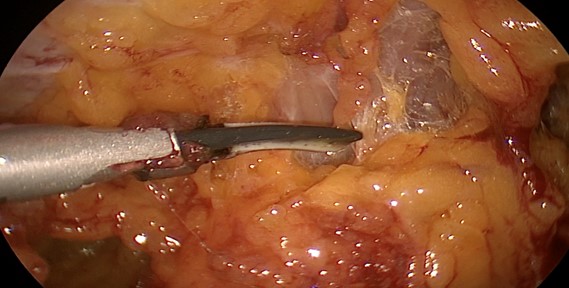
4: Bipolar Forcep
Bipolar forceps,( Fig. 4) atraumatic grasper, and vessel sealer are ideal instrumentation for small bowel resection. Harmonic Scalpel and or scissors can replace vessel sealer as needed. Needle drivers are necessary to close enterotomy. With the surgeon’s experience forceps can replace needle drivers.
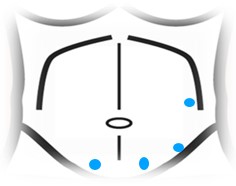
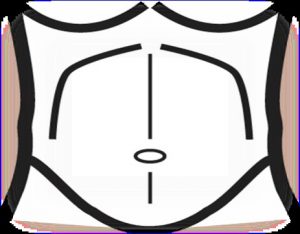
153.2.1 Trocars Placement
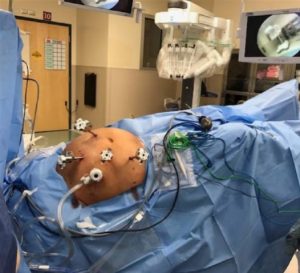
Fig. 5: Trocars Placement
Safe intra-abdominal access is the first step in minimally invasive robotic surgery. Sites of previous operative intervention will certainly influence the strategy to gain initial access. Access to the peritoneal cavity is gained using different techniques including; Hasson Optic trocar and Veress needle. After adequate pneumoperitoneum is established, a 5-mm trocar is placed in the lateral position. It is critical to place the ports as far as possible to the middle line, allowing an increased range of motion and effectiveness. The midaxillary line should be the ideal placement for lateral trocars. ( Fig. 5)
Three or 4 robotic arms are used. An 8 mm trocar is placed in the lower lateral abdomen and the initial 5mm optical trocar is then replaced with an 8 mm trocar or by the camera trocar.( Fig. 6)
For terminal and distal ileum, port placement should be left lower quadrant, left middle quadrant, and subxiphoid. For duodenal lesions, port placement may vary.
The jejunum and proximal ileum, trocar placement is lateral midaxillary line either left or right. This will depend on the working site. Double docking can be placed at the subxiphoid or suprapubic area), allowing the surgeon to have a multipurpose trocar. (Fig 7)
153.2.2 Docking
The patient position must be performed prior to the docking of the robot. The robotic cart is driven directly towards the abdomen and over the trocar sites. The robotic docking is done from the side of pathology, to align the center column of the robot with the target and the camera. Patient on supine positions, arms tucked and table tilt contralateral to pathology. Trendelenburg or Reverse Trendelenburg will be determined by surgeon preference.
For terminal ileum and distal ileum, the Trendelenburg position is key to allow better visualization and instrumentation. For pathology over duodenum and proximal jejunum consider reversing Trendelenburg.
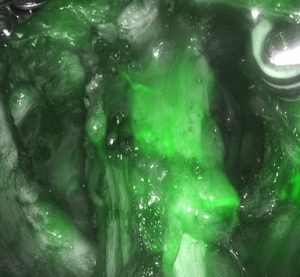
153.2.3 NIR fluorescence imaging
The association of ICG through hydrophobic interactions with lipids and polymers, and through electrostatic interactions with hydrophilic polyethylene glycol, potentially enhancing its stability and fluorescence, has been previously reported with liposomes and micelles.2.
ICG should be used after dividing the mesentery of the small intestine to identify proper vascularization. After identifying proximal and distal margins. The stapler can be used to divide the specimen.
153.3 Postoperative Care
Contemporary General and colorectal surgery is often associated with long lengths of stay. An enhanced recovery protocol (ERP) is a set of standardized perioperative procedures and practices that is applied to all patients undergoing a given elective surgery. In general, these protocols are not intended for emergent cases, but components of them certainly could apply to the emergent/urgent patient. Also known as fast-track protocols or enhanced recovery after surgery (ERAS) protocols, the content of these specific protocols may vary significantly, but all are designed as a means to improve patient outcomes
1. Patient Mobilization
Early and progressive patient mobilization is associated with shorter length of stay as well as DVT prophylaxis.
Within enhanced recovery programs (ERPs) for bowel surgery, definitions of early mobilization vary, from any mobilization at all within 24 hours to 8 hours per day by postoperative day.
2. Ileus Prevention.
Patients should be offered a regular diet immediately after elective bowel surgery. Multiple randomized studies, meta-analyses, and observational studies demonstrated that early (< 24h) feeding accelerated gastrointestinal recovery and decreased the hospital length of stay.
Sham feeding (i.e., chewing sugar-free gum for ≥10 minutes 3 to 4 times per day) after bowel surgery is safe, results in small improvements in gastrointestinal recovery, and may be associated with a reduction in the length of hospital stay.
Multiple systematic reviews and meta-analyses have been published, reporting that adding chewing gum to standard postoperative care was associated with significantly earlier time to flatus and bowel movement than those having ordinary postoperative treatment, with no significant improvement in postoperative complications, readmission, or reoperation rates.
The results wit some medicaments showed the accelerated time to recovery of gastrointestinal function doses compared with placebo and a significantly shorter hospital length of stay.
3. Postoperative Fluid Management
Intravenous fluids should be discontinued in the early postoperative period after recovery room discharge.
Intravenous fluids should be discontinued in the early postoperative period (after recovery room discharge) and clear fluids (≥1.75L/d of water) encouraged as tolerated soon after surgery. Intravenous fluids should be administered only when deemed clinically necessary. To prevent excessive fluid administration, daily postoperative weight gain should be monitored and weight gain >1 to 2 kg avoided.
4. Urinary Catheters
– Urinary catheters should be removed within 24 hours.
Urinary catheterization is routinely used in abdominal bowel surgery for intraoperative bladder decompression and monitoring of urinary output. Patients who undergo urinary catheterization for >2 days have twice the risk of a postoperative urinary tract infection.
REFERENCES
1.- Rodriguez, J. Laparoscopic and robot-assisted laparoscopic digestive surgery: Present and future directions. 2016.
2.- Clatterbuck, Brant, and Lane Moore. “Bowel, Small Resection.” In StatPearls. Treasure Island (FL): StatPearls Publishing, 2018. http://www.ncbi.nlm.nih.gov/books/ NBK507896/.
3.- Philipp-Alexander Neumann, Emile Rijcken. Minimally invasive surgery for inflammatory bowel disease: Review of current developments and future perspectives. 2016.
4.- Kraft, John C., and Rodney J. Y. Ho. “Interactions of Indocyanine Green and Lipid in Enhancing Near-Infrared Fluorescence Properties: The Basis for Near-Infrared Imaging in Vivo.” Biochemistry 53, no. 8 (March 4, 2014): 1275–83.
5.- Carmichael, Joseph, M.D. Keller, Deborah M.S., M.D. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery from the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017; 60: 761–784
6.- Gustafsson UO, Scott MJ, Schwenk W, et al.; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37:259–284.
7.- Nygren J, Thacker J, Carli F, et al.; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:801–816.